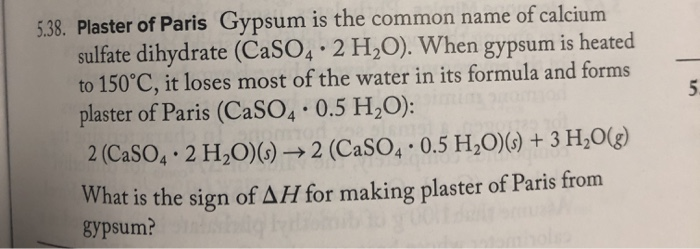Formation and Transformation of Five Different Phases in the CaSO4−H2O System: Crystal Structure of the Subhydrate β-CaSO4·0.5H2O and Soluble Anhydrite CaSO4 | Chemistry of Materials
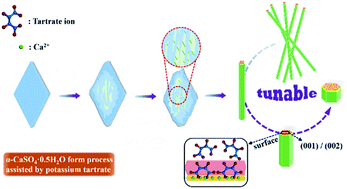
Aspect ratio-controlled preparation of α-CaSO4·0.5H2O from phosphogypsum in potassium tartrate aqueous solution - RSC Advances (RSC Publishing)
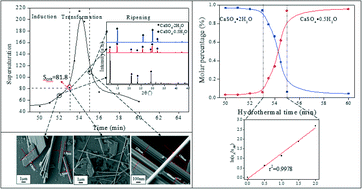
Supersaturation-induced hydrothermal formation of α-CaSO4·0.5H2O whiskers - CrystEngComm (RSC Publishing)

SOLVED: Gypsum is the common name of calcium sulfate dihydrate which has the formula CaSO4· 2 H2O When gypsum is heated to 150^∘C, it loses most of the water in its formula

The effect of additives on the hydration of CaSO4·0.5H2O: A synchrotron X-ray diffraction study - ScienceDirect

PDF) Bassanite (CaSO4·0.5H2O) dissolution and gypsum (CaSO4·2H2O) precipitation in the presence of cellulose ethers

Influence of the Process Parameters on the Formation of CaSO4·0.5H2O Whiskers – topic of research paper in Nano-technology. Download scholarly article PDF and read for free on CyberLeninka open science hub.

Crystal structure of (a) γ‐CaSO4 and (b) bassanite CaSO4 ⋅ 0.5H2O, as... | Download Scientific Diagram